Thinking about the science behind Lumina Probiotics
Lumina probiotics, per their company website, “is a probiotic optimized through biodesign to rebalance your oral pH and inhibit the growth of acid-secreting bacteria, to protect enamel and freshen the mouth”. It is marketed as an anti-cavity product. How does their product work? And what science backs up their work?
First, a high-level overview of cavities and periodontal disease
Tooth demineralization by acids produced from bacterial metabolism in the mouth lead to cavities. First, bacteria (most often Streptococcus mutans) adhere to the tooth forming biofilms or plaque. Then, they metabolize carbohydrates which produce organic acids. This acid lowers the pH of the mouth leading to calcium and phosphate leach out of the tooth leading to cavity formation. Bacteria can colonize the dentin or pulp causing structural damage and pain.
Periodontal disease starts with biofilm production on the tooth surface from bacteria like Porphyromonas gingivalis. These bacteria secrete virulence factors which activate the immune system. Chronic inflammation leads to differentiation of osteoclasts and suppression of osteoblast activity which promotes bone loss and impairs bone formation.
What is the science behind Lumina Probiotics?
Their product is based on this paper from 2000 (Hillman 2000). This paper aims to introduce a harmless strain of bacteria into the mouth such that it colonizes the mouth and prevents outgrowth of the pathogenic Streptococcus mutans.
1) A naturally occurring strain of Streptoccocus mutans (JH1000) was found to produced mutacin 1140, an antibiotic capable of killing most but not all strains of streptocci (see below; taken from Hillman 1984). Notably, this antibiotic is not sensitive to bacteroides gingivalis (Porphyromonas gingivalis), and subtypes of streptococcus sanguis and faecalis. This antibiotic, by killing many of the Streptoccocus species but not the Porphyromonas species, may result in rampant growth of Porphyromonas – the primary causative agent in periodontal disease. The same logic applies to sanguis and faecalis, both of which are known pathogens in chronic endodontic and periodontic infection.
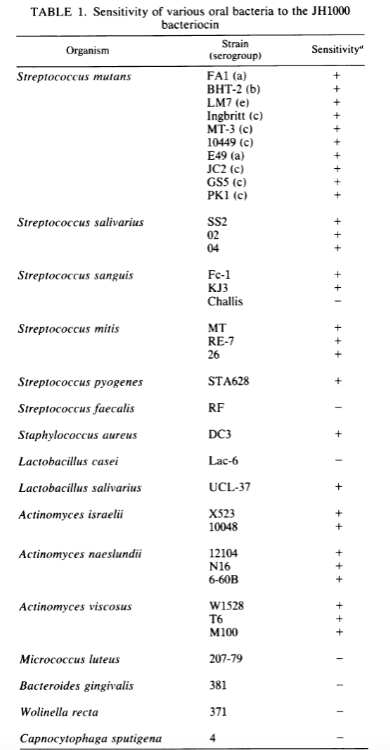
2) Pathogenic Streptoccocus strains are acid producers which create the proper pH environment for cavity formation. Thus, Hillman seeks to design a strain incapable of making (lactic) acid. Hillman found that removing the enzyme necessary for lactate production is lethal to Streptococcus mutans likely due to the buildup of toxic metabolic byproducts that are unable to be recycled. But, this lethality was overcome by upregulating the alcohol dehydrogenase activity intrinsic to S. mutants. Ethanol is now created instead of lactic acid. Lactic acid is considered cariogenic but ethanol is not technically considered cariogenic. However, oral bacteria can produce acetaldehyde from ethanol which is a known carcinogen.
Hillman combines both (1) and (2) to create a S. mutans strain that produces ethanol over lactic acid and secretes an antibiotic that removes some of the oral pathogens in the oral cavity. S. mutans was likely used since we already know that it colonizing the mouth. This strain of S. mutans with both changes is called “BCS3-L1”. Lumina sells BCS3-L1.
BCS3-L1 infected rates had a caries score that was ~50% lower than JH1140 (antibiotic-only). S. mutans-free rats had similar caries scores to BCS3-L1 infected rats. There were 10 rats used in each group. There is no control group demonstrating the caries score for rats without modifications so it is hard to gauge the effect size of BCS3-L1.
The caries score is taken from a 1958 paper where the linear extent of a lesion in one plane is judged by eye and the depth of penetration is recorded. Depth is given either a 1, 2, 3, of 4 value based on extent of penetration and are summed together. This method does not consider the clinical impact of a lesion, or the surface area of the lesion. It treats the depth as an ordinal variable but sums it together like a continuous one.
Rats were inoculated with a cotton tip soaked with bacteria and moved side to side two-times in the oral cavity for 15 seconds each at 24 hour intervals. This method was shown to result in colonization after 3 weeks in rats and were able to displace other strains. The rats were given the bacteria at 21 days of age likely before they had significant dental damage. They used Sprague-Dawley rats who live for 24 months on average. So, infection at 21 days is equivalent to infecting a human who will live for 75 years at 2 years old.
Conclusion
This company is based on a paper with low sample sizes, loosesly defined measurement approaches, and unclear effect sizes. Further, based on their reported data, there is a decent possibility of creating severe periodontal disease by using this product. Specifically, by introducing an antibiotic to which key periodontal and endodontal pathogens are resistant, these pathogens can grow relatively unchecked.
More so, the efficacy of preventing cavities is not clear given the opacity of the caries score they use. There is a lack of a true control group with normal oral microbes in their studies. Further, rats are not humans. Humans drink water, soda, eat different types of food, sleep with their mouth open, and already have different stages of dental disease that 21 day old rats (~2 year old humans) do not.
The oral microbiome regulates diseases of the mouth but also extra-oral diseases like diabetes, cancer, and obesity. Oral products should be supported by solid mechanistic evidence and human data.
This is an interesting concept but is not quite there right now.
As always, happy to take feedback on this take.