HIV Point of Care (PoC) early detection and monitoring with RT-LAMP
Summary
Broadly accessible PoC finger prick RT-LAMP HIV detection tests more sensitive and specific than currently offered PoC antigen/antibody immunoassays can now be developed and commercialized. These tests can be offered direct-to-consumer, in jails, clinics without lab access to PCR equipment, and countries with limited resources (e.g., Sudan).
With some R&D, a viral load RT-LAMP assay can likely be developed for monitoring response to therapy - monitoring disease is typically a larger market than early detection. Likewise, the Athelas One device can potentially be used to estimate CD4+ T cell count, another important HIV monitoring metric.
Any other DNA or RNA viruses can be detected using the RT-LAMP/LAMP platform by simply loading a different virus-specific shelf-stable primer. A suite of PoC viral detection products can readily be created.
Overview
Approximately 1.2M people have HIV and 13% of these people are unaware of their diagnosis and need testing (1). In 2020, 30,635 people received an HIV diagnosis in the US with the highest rates of new diagnoses occurring in the South (1). Worldwide, the majority of HIV cases are HIV-1, with HIV-2 largely occuring in West Africa. HIV-1 has three main groups, M, O and N with the M subgroup dominating. There are 9 M subtypes of which the B subtype is most prevalent in the US, Europe, and Australia (2).
For screening, the CDC recommends that everyone aged 13-64 receive at least one HIV test annually and those at higher risk (e.g., men who report male-to-male sexual contact) are tested more frequently. Current screening tests include OraQuick (3), a PoC oral swab antibody test, and mail-in tests requiring a small sample of blood from a finger prick that looks for anti-HIV antibodies (4). In low resource settings (e.g., parts of Africa) with limited healthcare staff or equipment, both rapid diagnostic tests (PoC antigen or antibody tests) and dried blood spots are used (5). Dried blood spots are sent to laboratories for nucleic acid and antigen/antibody testing and implementation of this technology for HIV viral load quantitation is considered one of the most medically effective immediate measures to reduce AIDS-related deaths in Africa (5, 6).
Broadly, there are two areas in which implementing new approaches can help improve outcomes: a) early detection of HIV infections, b) monitoring HIV viral load for managing treatment.
The primary technology to implement in both of these cases is RT-LAMP (reverse transcriptase loop mediated isothermal amplification). LAMP is a DNA amplification method that allows for rapid and sensitive detection of a specific gene. Reverse transcriptases are needed when working with RNA viruses like HIV and COVID19 to convert their genetic material from RNA to DNA before amplifying. RT-LAMP offers a simple, scalable, and accessible testing method for detecting viral RNA with performance on par with RT-PCR (7).
Early detection of HIV infections
HIV RNA in plasma spikes during acute infection, and stays at a significant non-zero level thereafter (Figure 1, h/t Dr. Sheldon Campbell YNHH adapted from (8)). Other antigen and antibody markers are detectable at later stages and drop off quickly (p24 Antigen) or peak later in post-acute stages (IgM, IgG) making them less ideal markers. Detecting HIV RNA is the gold standard but is accomplished clinically via RT-PCR, a method requiring specific laboratory equipment and a mechanism to cycle temperatures (9). RT-LAMP is an alternative to RT-PCR that does not require special instruments.
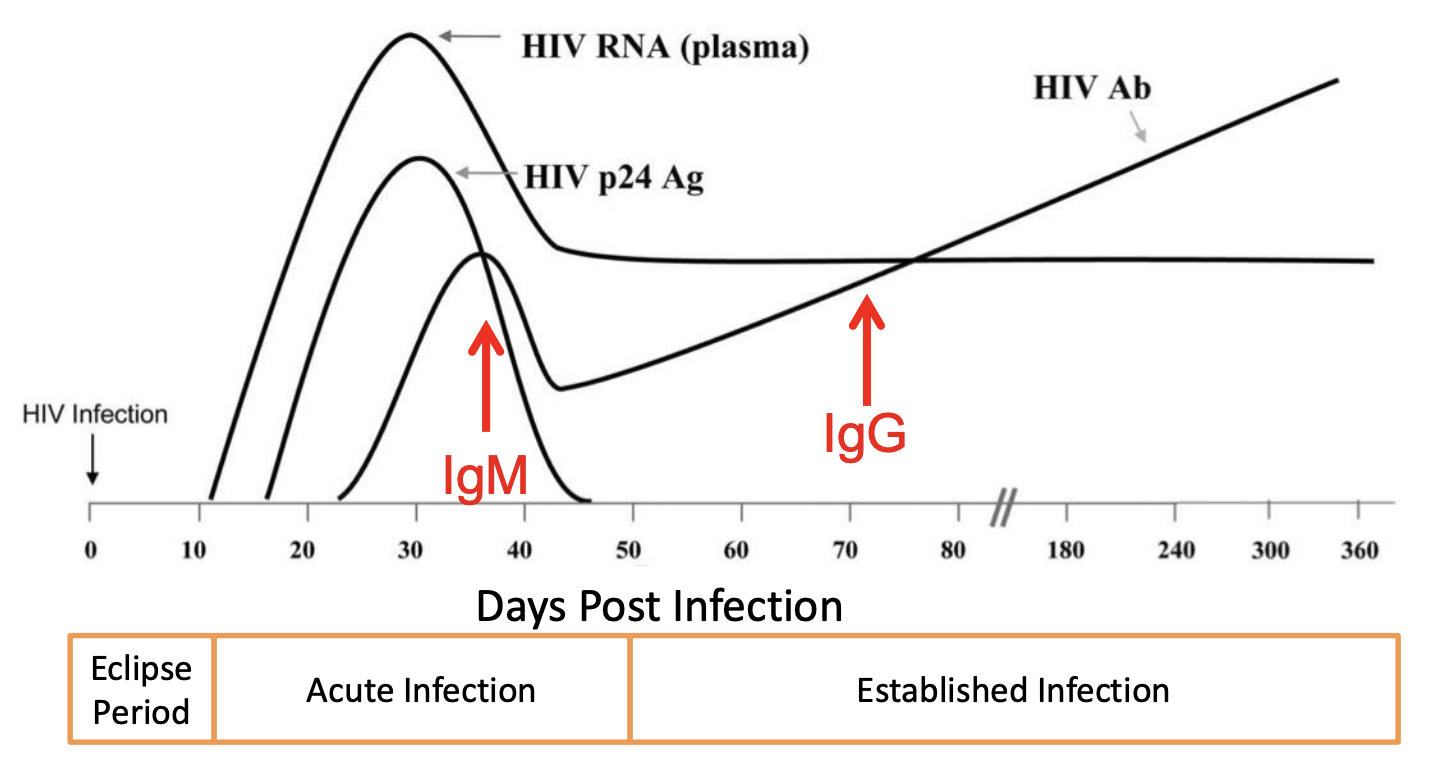
Figure 1 h/t Dr. Sheldon Campbell YNHH adapted from Ref. 8, longitudinal profiles of HIV biomarkers
A colorimetric RT-LAMP assay has been used for PoC detection of COVID19 (10). Detect commercialized a RT-LAMP test for detecting COVID19 from an anterior nasal swab with 91% sensitivity and 100% specificity. For proper use, the patient must perform a few specific maneuvers to ensure valid results which may make it more difficult for people to use compared to standard antigen tests with simpler procedures (11). The test tube loaded with the patient sample is processed by the Detect Hub (the small pod that performs the RT-LAMP assay) in an hour and results are read and tracked by their app. The Detect COVID19 test demonstrates the feasibility of the RT-LAMP as a PoC detection tool.
The market for a RT-LAMP HIV PoC detection test in the US exists in offering a home-based test, and distributing tests to limited resourced areas like jails or through mobile testing clinics. The current algorithm for HIV diagnosis in hospitals begins with an antigen/antibody test and then either a nucleic acid test or further antibody differentiation assay. This approach requires multiple tiers of testing, and in clinics without access to large platform nucleic acid testing laboratories, it may be appropriate to begin with RT-LAMP due to its speed, accuracy, portabilitiy, and low cost. A HIV-1 RT-LAMP assay detected seroconverting individuals 1-3 weeks earlier than a rapid HIV antibody test and up to 2 weeks earlier than lab-based antigen/antibody immunoassays (12).
RT-LAMP for HIV detection has currently been studied with plasma drawn from a vein (12-15). There is no evidence suggesting HIV RNA can be detected via RT-LAMP with saliva, though this is likely the most ideal sample to collect because it is the most non-invasive and requires the least equipment. OraQuick, the current competitor to a RT-LAMP PoC test, uses an oral gum swab. Thus, engineering an oral swab-capable assay will position a RT-LAMP product more competitively in the PoC HIV testing landscape. However, the next best sample type would be whole blood obtained from a finger stick. Finger stick blood (10 microliters) has been successfully used in an RT-LAMP proof of concept (16) suggesting that results will not differ significantly between finger prick and venipuncture RT-LAMP assays.
Outside of the US, low resource settings with increased HIV incidence due to a lack of testing and laboratory materials may benefit from an accessible, sensitive, and specific HIV RT-LAMP detection test. The efficacy of such an assay has already been demonstrated in a Sudanese population (n=90). RT-LAMP performed as well as RT-PCR with a high negative predictive value (NPV) indicating that people without HIV rarely if ever tested positive (13). A portable hand-held hub capable of performing the RT-LAMP assay could process finger prick tests in an hour and greatly increase testing coverage in countries like Sudan.
Both within and outside the US, a single hub analyzing samples coupled with a finger prick stick and test tube collection system could process a sample every hour. Linked to a mobile application, test results can be distributed to patients and resources can be coordinated from this app.
Monitoring of HIV Viral Load
Antiretroviral therapy (ART) successfully prevents HIV positive patients from spreading the virus, developing advanced symptoms like end organ damage and neurological complications, progressing to AIDS, and constantly contracting infections due to a weakened immune system (17, 18). ART is monitored and adjusted based on HIV viral load and CD4+ T cell count (19). Repeat viral load measurement should be performed at 4 to 8 week intervals until the levels fall below PCR limit of detection and if viral suppression is not possible, viral load should be monitored every 3 months or more frequently (20).
Currently, HIV viral load quantitation involves a venipuncture blood draw at either an outpatient clinic or hospital followed by RT-PCR. Results require costly equipment, slow turnaround times, and patients must travel to give blood. An RT-LAMP PoC assay for viral load quantitation can remove these barriers.
To date, however, a RT-LAMP assay capable of estimating viral load with decent accuracy has not been developed. A two-step RT-LAMP assay was developed and tested on four patient samples, and it produced markedly different estimates from consensus RT-PCR (Figure 2 - directly taken from Figure 4B of ref. 15). A finger-prick ready RT-LAMP assay must be engineered to capitalize on the market for monitoring response to therapy for HIV.
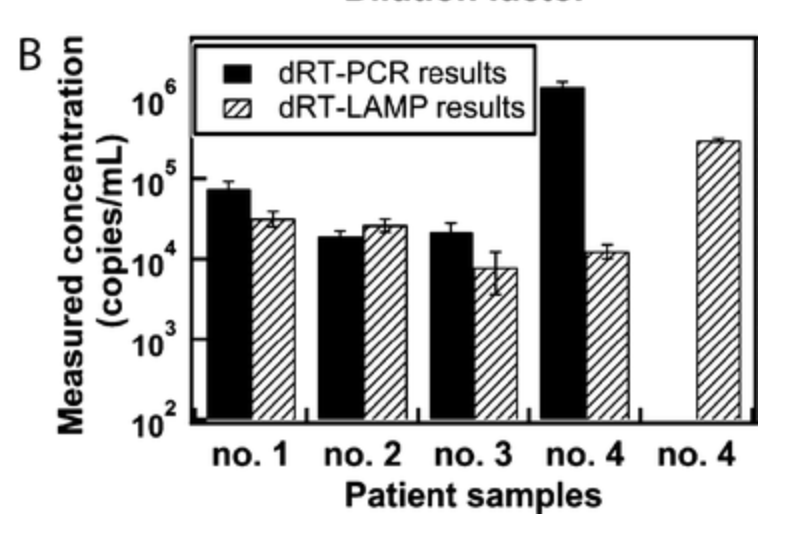
Figure 2, taken directly from Fig 4B of Ref. 15
Monitoring of CD4+ Count
CD4+ T cell count monitoring is usually recommended every 3 to 6 months and used to monitor HIV progression (21, 22). The Athelas One is a PoC device made of a single head light microscope that analyzes a finger stick of blood to report total white blood cell (WBC) and absolute neutrophil count (ANC) (23, 24). Currently, total WBCs are measured but with further development and training of computer vision algorithms it may be possible to use the existing hardware to count CD4+ T cells.
To assess the feasibility of CD4+ T cell counting with the current hardware, the following analysis could be performed. Collect a finger prick blood sample from a number of patients and matched venous blood draws. Stick the sample under the Athelas One light microscope and store that image. Now, stain the sample for CD4+ T cells and count them to obtain a gold standard estimate. Return back to the Athelas One image and label on the image where the CD4+ T cells are located. Then, train a machine learning model to identify and count CD4+ T cells. The two factors that will determine if this project is worthwhile to commercialize: a) model performance in CD4+ T cell counting, and b) an assessment of whether the CD4+ T cells sampled from a finger prick are an accurate marker for venous blood draw CD4+ T cell count.
Extending RT-LAMP To Other Disease Conditions
The LAMP/RT-LAMP system can be easily extended to detect other viral diseases in a PoC setting. A specific LAMP primer must be made for each virus to be detected, but otherwise the entire system remains the same. Of particular note, the shelf stability of LAMP primers under different temperatures, specifically normal kitchen refrigeration and room temperature, are important to consider. Some data suggests that LAMP primers are stable for nearly 50 days and across a wide range of temperatures (25). Investing into designing primers that are more shelf and temperature stable is likely important if multi-viral detection LAMP PoC assays are to be commercialized.
Thus, a suite of viral finger prick PoC testing products can be created and offered direct-to-consumer. The customer must make a one-time purchase of a hub where the LAMP/RT-LAMP reaction occurs and then can order primers for any viral diseases of interest.
Adith Arun
References
1 https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics#:~:text=Fast%20Facts
2 https://en.wikipedia.org/wiki/Subtypes_of_HIV
3 https://oraquick.com/what-is-oraquick
4 https://www.cdc.gov/hiv/basics/hiv-testing/hiv-self-tests.html
5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7075356/
6 https://pubmed.ncbi.nlm.nih.gov/26633768/
7 https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00530-2/fulltext
8 https://pubmed.ncbi.nlm.nih.gov/21406978/
10 https://www.science.org/doi/10.1126/scitranslmed.abc7075
12 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4439053/
13 https://academic.oup.com/ajcp/article/154/Supplement_1/S138/5942327
14 https://pubmed.ncbi.nlm.nih.gov/29474813/
15 https://pubs.acs.org/doi/10.1021/ac3037206
16 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3285652/
18 https://www.plannedparenthood.org/learn/stds-hiv-safer-sex/hiv-aids/what-are-symptoms-hivaids
19 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6892619/
21 https://pubmed.ncbi.nlm.nih.gov/15817962/